


Almost thirty years after its publication, it was necessary to review this legislation on Personal and Protective Equipment (PPE) for mainly organisational and political reasons:
Furthermore, the experience gained over these thirty years had enabled organisational and technical improvements to be highlighted that it was important to implement.
The PPE Directive is now unanimously recognised as one of the successes of the new European approach launched in 1992. This text has enabled an improvement in the protection of individuals in their workplace, and the standardisation of this pro-tection across all member States, thus allowing very significant progress to be made in numerous countries. A single market was created and the free circulation of PPE throughout Europe enabled companies in the sector to develop. Many products offering mediocre protection were able to be removed with the obligation to comply with essential health and safety requirements. It is for these varied reasons that the new regulation should not call into question the general philosophy of the directive, and in particular the heart of the text, appendix 2: the essential health and safety requirements are almost identical in both texts. Furthermore, the PPE classification certification rules have not changed (see 1).
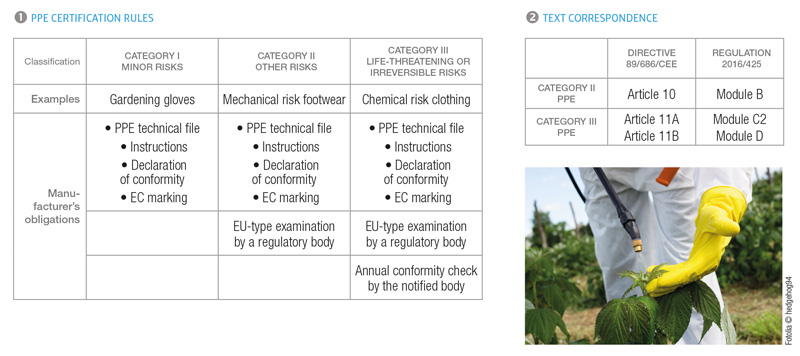
From a point of view of the certification of protective gloves, footwear, and clothing, the main changes to be taken into account between directive 89/686 and regulation 2016/425 for manufacturers are:
In addition to these changes, it is clearly necessary to review product instructions (reference to the regulation, possible expiry date, website for the EU declaration of conformity, etc.).
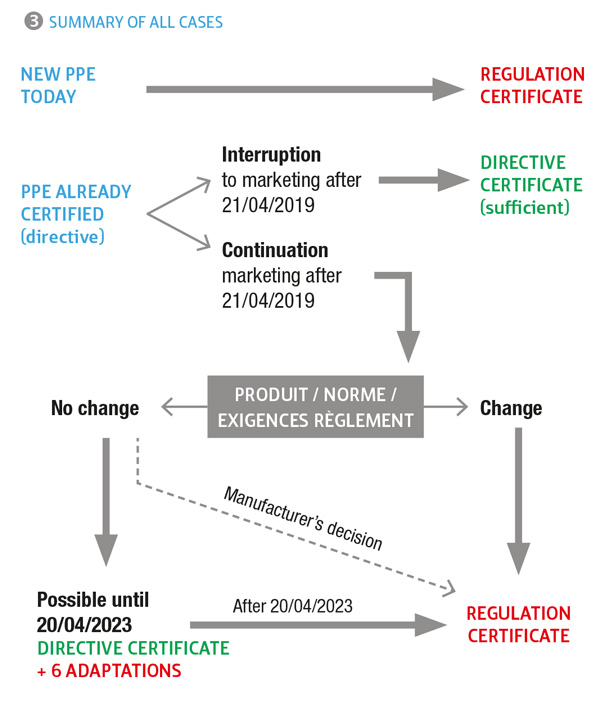
For new products, the requirements of regulation 2016/425 on the certification of PPE are applicable to products placed on the market after 21 April 2018. Certificates based on the directive may also be issued until 20 April 2019 (not recommended!). After this date, only the regulation will be applicable. For products certified under the directive and already on the market, it will be necessary to transfer their certification under the regulation. Tens of thousands of items of PPE are concerned, therefore the workload for manufacturers and regulatory bodies is considerable. In order to limit the scope of the re-certification work, the Commission has envisaged two scenarios.
Certain products certified under the directive may benefit from a simplified certification procedure under the regulation. This is the case of products for which:
In this event, the manufacturer files their transfer requests with the regulatory body, which will convert their EC certificate without carrying out any new tests.
In accordance with the document issued by the European Commission in December 2017, some products certified under the directive may continue to be marketed until 20 April 2023 with their initial certificate if:
In the event of testing at the request of the authorities, the manufacturer must demonstrate that it complies with these points. Figure 3 enables all cases to be summarised.
The manufacturer must, therefore, issue EU declarations of conformity under regulation 2016/425/EU based on an EC certificate under directive 89/686/EC.
For “own-brand” certificates, we used to issue extensions to the directive in minor variants. Under regulation 2016/425, it is the original manufacturer who will introduce the certification request for the secondary manufacturer, from their technical documentation and subject to an agreement between the two parties. CTC will then issue certificates on behalf of the secondary manufacturer. The initial technical documentation will be sent to the regulatory body with the changes related to the secondary manufacturer: example of marking and instructions. The elements will be retained by the regulatory body, which may send them to the competent authorities upon specific request.
The current situation is critical. Numerous manufacturers have waited for the new regulation in order to launch new products, and requests to transfer from the directive to the regulation are very high in number. Consequently, all regulatory bodies have extremely busy certification schedules, and the jurisprudence has not yet been established. The European Commission is still developing its position. We would also note that the interpretations from one member State to another may sometimes differ.
CTC was one of the first PPE regulatory bodies for the directive, and it has awarded more than 30,000 certificates in accordance with this framework. This experience is well known and, since 30 November 2017, CTC has also been notified under regulation 2016/425 for module B (EU-type examination) and module C2 (conformity with type based on the internal control of manufacturing and supervised controls of the product at random intervals). Our Certification division is fully mobilised to provide its services and support to PPE manufacturers of protective gloves, footwear, and clothing.
|
ECONOMIC OPERATORS
|